
Life Science Newsletter
The following is included in this e-tech newsletter:
- Transdermal dosage forms via twin screw extrusion - Making lives better!
- Flood feeding extremely small batches on a co-rotating intermeshing twin screw extruder (TSE)
- Comparison of binder addition sequencing for a melt granulation formulation
- Spray drying versus melt extrusion for amorphous solid dispersions
- Univ. TX Austin installs ZSE-12 twin screw extruder into PharmE3D process laboratory
- Leistritz expands USA process development laboratory
- Plant based protein extrusion capabilities installed @ Leistritz process laboratory
- Pharmaceutical Extrusion Technology Textbook, 2nd edition
Transdermal drug delivery systems - making lives better!
Transdermal products are being produced via extrusion as alternatives to more classic drug delivery systems. Transdermal delivery is a non-invasive method of drug delivery where the medication is delivered into the body over a period of time through the skin.
These systems typically contain adhesive material, rate-controlling membrane, backing materials, reservoir vehicle, and a release liner. In the reservoir and matrix type systems the adhesive component keeps the patch on the skin and has no further function. In the drug-in-adhesive system, the drug is actually incorporated into the adhesive and is much more critical to the overall function of the system. Various structures are depicted below.
Example of various transdermal structures possible via continuous extrusion/lamination
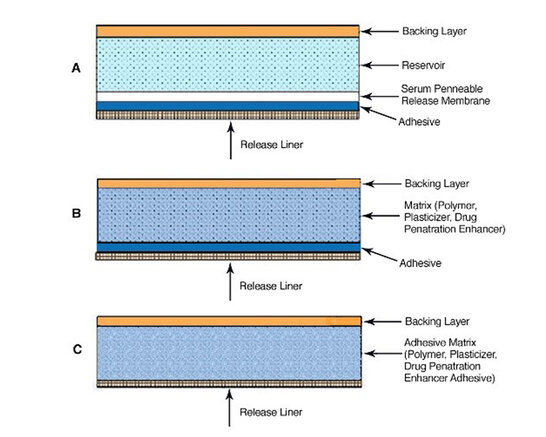
Drug-in-adhesive formulations have been manufactured via solvent casting, but melt extrusion provides several advantages. Solvent casting involves long processing time, high costs, and environmental concerns. Comparatively, extrusion is a continuous, cost effective mixing process that does not use solvents, and can also increase solubility, absorption, and efficacy of poorly soluble drugs through the creation of a solid dispersion. Extrusion systems are not only limited to creating the drug matrix and drug-in-adhesive, but also the complete structure.
A typical transdermal system includes the following devices:
- Loss-in-weight feeders to meter excipients and APIs into the TSE
- Specialty extruder to condition and meter a pharmaceutical grade rubber into a TSE
- Gear pump to build and stabilize pressure to a film die
- An in-line gauge to monitor extrudate thickness
- Lamination system to form and wind the transdermal structure
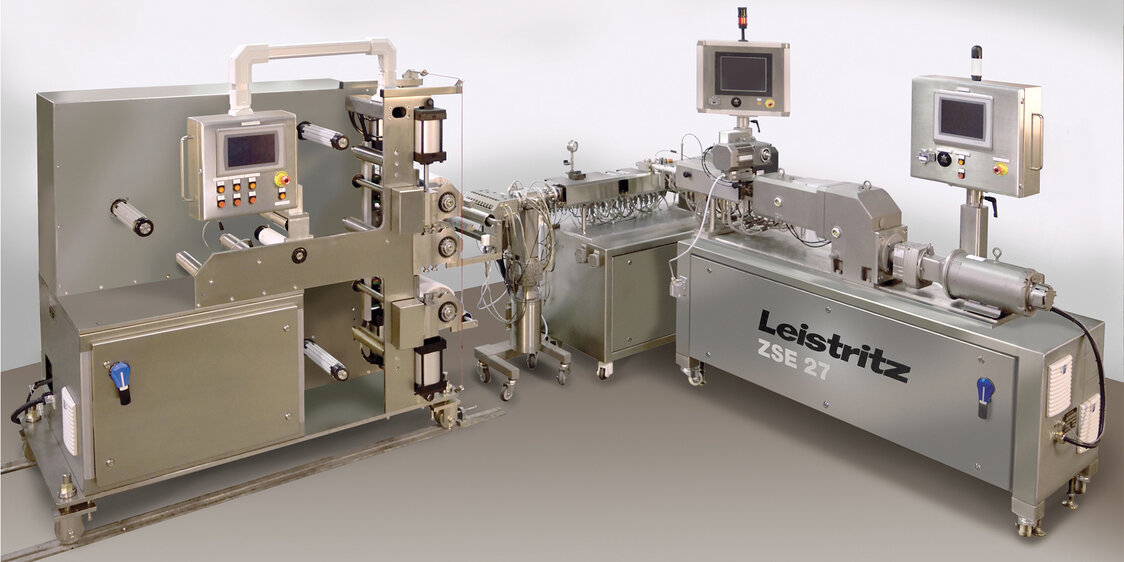
ZSE-18 transdermal system with specialty rubber extruder
Schematic of a downstream lamination system
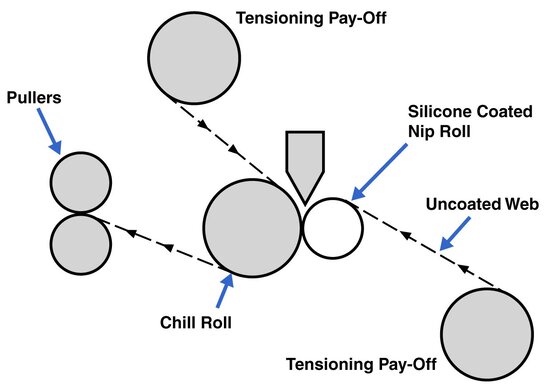
The web path is the route the material and any substrate will take from the die exit to the end of the system. In designing this path, it is important to consider the properties of the product as it moves through the path. Various configurations are available based on the materials and final structure.
For a white paper describing the die and downstream equipment to manufacture transdermal products CLICK HERE.
Flood feeding extremely small batches on a co-rotating intermeshing twin screw extruder
Extruders are either flood fed or starve fed. Flood feeding is typically used in single screw extrusion, where the feed hopper is filled and the extruder screw rpm determines the feed rate. Starve feeding is the standard practice for twin screw extrusion, where a separate feeder meters APIs/excipients to the TSE, and the TSE screws rpms is set independently to optimize processing efficiencies.
Flood feeding on a TSE is atypical because it will normally over-torque the extruder. To overcome this limitation a ZSE-16 mm TSE with low free volume (1 mm flight depth) and high-torque splined shafts (72 NM torque) was used.
Early stage pharmaceutical processes often require minute quantities of materials to be processed. There have been challenges evaluating small batches at these low quantities, as auger style feeders often require 200+ grams of material to reach equilibrium. Flood feeding a twin-screw extruder has previously not been evaluated to determine viability due to torque limitations. However, the shallow flight depth (1 mm) and high torque capacity of the ZSE-16 mm TSE and specialty feed section of the screw design facilitates successful flood feeding.
A study was presented at AAPS 2020 implementing this novel technology. Specialty feed screw elements that limit the intake of material followed by high-forwarding efficiency screw elements were specified to effectively simulate a starve fed process and to maintain the mixing efficiencies associated with varying degrees of screw fill. A series of 50 gm batches were processed utilizing the flood fed method on a ZSE-16 with nearly full batch utilization for the test samples. In general (and after processing) the product was amorphous and the color indicated minimal, if any, degradation.
The flood feeding method described enables a quick and efficient approach to process small volume pharmaceutical batches via melt extrusion. The modified/atypical screw design effectively simulates a starved fed TSE system and allows extrusion samples to be produced with near full batch utilization, as sample wastage associated with auger style feeders is eliminated. It is evident that the flood fed ZSE-16 system offers a considerable benefit when only limited API quantities are available.
A comparison of binder addition process sequences in a twin screw melt granulation process
As a solvent-free continuous process, twin-screw melt granulation (TSMG) is emerging as a robust processing method for improving the flowability and tablet-ability of powder blends. The intensive mechanical forces imparted by rotating screws during TSMG enables effective binder distribution, but also jeopardizes API stability. Various binder addition methods have shown to significantly impact granule growth and granule properties in batch melt granulation processes. However, the influence of binder addition methods on granule properties (and API stability) in TSMG has not been investigated. This study compares three binder addition methods in TSMG:
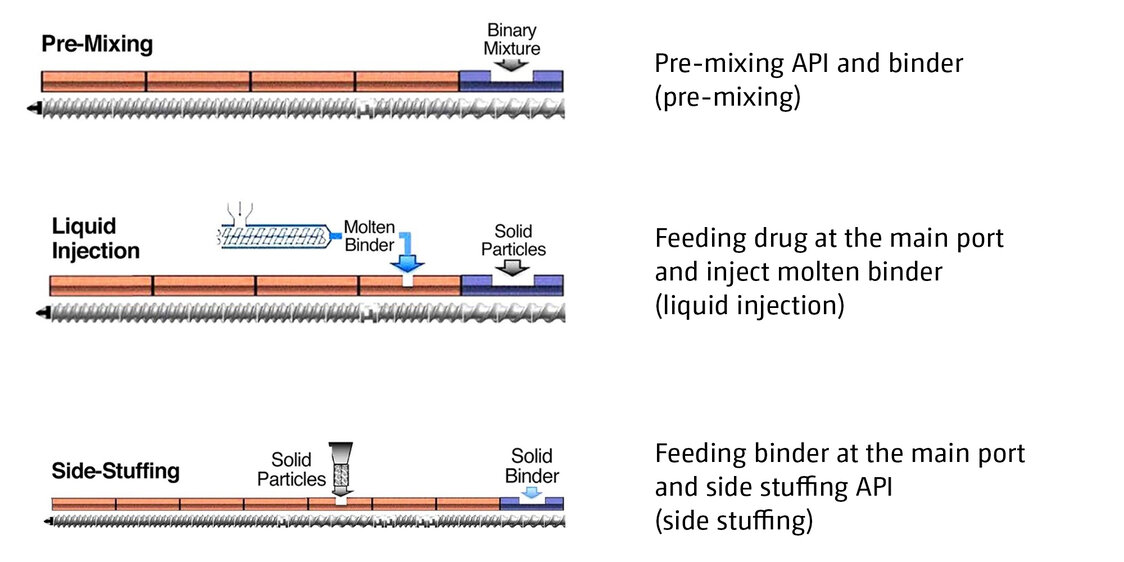
The effects of the different binder addition and sequencing on granule formation and stability of primary solid particles was presented at AAPS 2020. (the results might surprise you)
Spray drying (SD) vs melt extrusion (ME) as manufacturing processes for amorphous solid dispersions
Authored by Hibre Terefe and Isaac Ghebre-Sellassie, ExxPharma Therapeutics LLC
Amorphous solid dispersions of poorly soluble drug substances are prepared using a variety of technologies, the most popular of which are spray drying (SD) and melt extrusion (ME). SD and ME generate intermediate material that is subsequently used to develop a dosage form. Although both processes generate amorphous solid dispersions, the characteristics of the materials are different. SD generates porous materials of various sizes. ME generates materials that are dense and the range of particle sizes is determined by inline or offline milling process. Due to the porous nature of the particles, materials prepared by spray drying have low bulk density as compared to ME that generates materials with a high bulk density.
SD utilizes organic solvents to manufacture particles that often contain residual solvents and ME does not. The material during ME may contain a small amount of moisture that is dried by venting steam/volatiles during the ME process and the material generated is practically moisture-free. In both cases, the amorphous solid dispersions are hygroscopic and have the tendency to absorb moisture that would lead to crystallization unless moisture protective measures are taken.
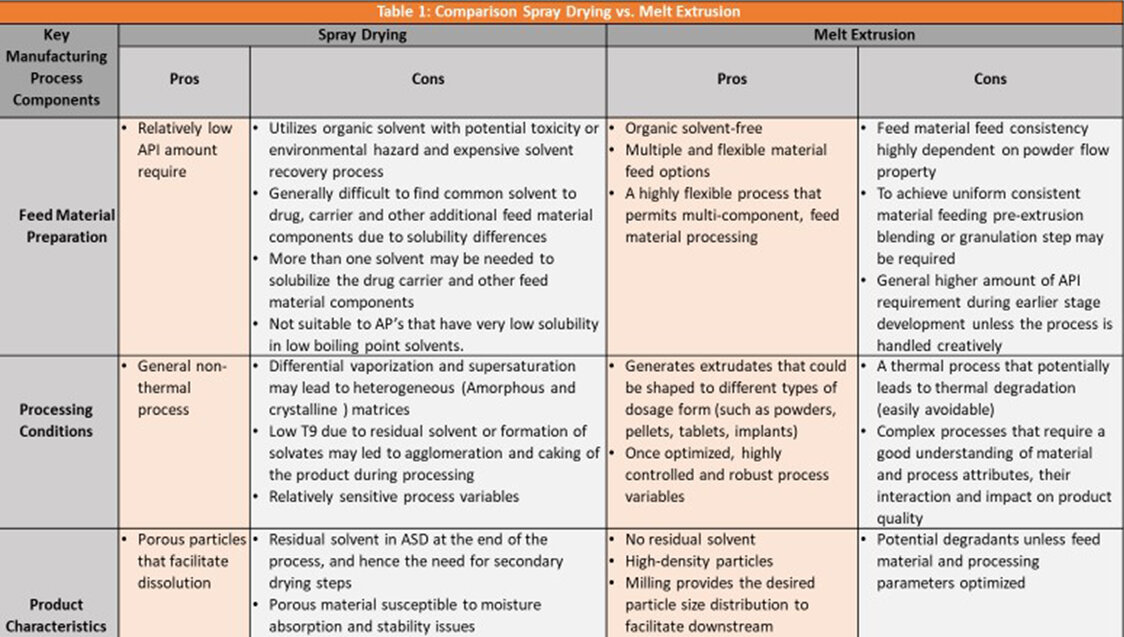
Spray drying and melt extrusion are both proven and accepted technologies to manufacture Amorphous Solid Dispersions. Before choosing, it is important to understand the pros and cons, level of complexity, scalability, and cost-effectiveness of the two manufacturing processes.
To download a two-part detailed article that compares these spray drying and melt extrusion technologies CLICK HERE.
Univ. TX Austin installs ZSE-12 into Pharmaceutical Engineering & 3D Printing (PharmE3D) Lab
3D printing has emerged as a versatile fabrication technology that utilizes polymers, APIs and excipients deposited in sequential layers to produce a prototype 3D object (i.e. pill or medical device). In 2020 a Leistritz ZSE-12 mm twin screw extruder with a downstream 3D filament system was installed into the new 3D lab at UT Austin under the auspices of Dr. Mohammed Maniruzzaman.
Image: ZSE-12 @ UT Austin 3D pharma laboratory
Work in Dr. Maniruzzaman’s lab focuses on pharmaceutical process engineering, continuous manufacturing and 3D printing of medicines. Additional projects focus on 3D bioprinting of scaffolds and smart medical implants, as well as ultra-portable drug delivery devices.
For additional information see: https://sites.utexas.edu/maniruzzaman/research/
Image: schematic ZSE-12 3D filament system
Leistritz expands USA process development laboratory
The Leistritz NJ USA process development laboratory has been expanded. Recent additions include a ZSE-12 twin screw extruder for small batch quantities, die and downstream equipment to produce 3D filaments.

ZSE-12 with plunger feeder and top feed capability

ZSE-18 with downstream equipment for 3D filament sampling
The Leistritz laboratory facility has wide ranging capabilities including, but not limited, to run the following trials:
- Melt extrusion, melt granulation and wet granulation
- 50 gm batch sample to 100+ kgs/hr
- Transdermal/laminate systems, including coex tooling
- Supercritical CO2 injection for foaming, stripping or as a fugitive plasticizer
- Mono-layer or coex rods or tubes, cut-to-length or wound on reels
- Wide range specialty formulations and parts
Contact Leistritz today to discuss the development of the next breakthrough dosage form!
Plant based protein extrusion capabilities @ Leistritz process laboratory
Based on the high success rate associated with manufacturing GMP dosage forms, many nutritional products are converting to high volume, continuous processing. Recent trends in the food industry have been moving towards plant-based protein sources in place of traditional animal protein sources. Utilizing twin screw extrusion technology, plant proteins (soy, lupin, pea, bean, etc.) can be transformed into meat alternatives. These texturized vegetable proteins (TVP) are produced using an extrusion process designed to mix, cook, and texturize the ingredients giving the feel and taste of meat, with little waste and less harm to the environment.
The Leistritz Extrusion Process Laboratory allows for the evaluation and production of the TVP and other nutritional processes. With a variety of solid feeders, liquid pumps, and dies the extrusion line can be designed specifically for a wide range of TVP and nutritional products.
See this link to a video on this subject: click here
Pharmaceutical Extrusion Technology Textbook, 2nd edition
Edited by Isaac Ghebre-Sellassie, Charles Martin, James DiNunzio and Feng Zhang
The first edition of Pharmaceutical Extrusion Technology, published in 2003, was deemed the seminal book on pharmaceutical extrusion. Now it is expanded and improved, just like the usage of extrusion has expanded, improved and evolved into an accepted manufacturing technology to continuously mix active pharmaceutical ingredients with excipients for a myriad of traditional and novel dosage forms.
Pharmaceutical Extrusion Technology (Second Edition, published in 2018) reflects how extrusion has spawned numerous research activities, in addition to hardware and process advancements. Expanded chapters contain all the extrusion related technical information necessary for the development, manufacturing, and marketing of pharmaceutical dosage forms.

Features include:
- Explanation as to why extrusion has become an accepted technology to continuously mix active pharmaceutical ingredients with excipients
- Focuses on equipment and process technology
- Describes various extrusion system configurations for a variety of dosage forms
- Presents new opportunities available only via extrusion and future trends
- Includes contributions from experts in the process and equipment fields
To review or to order Pharmaceutical Extrusion Technology Textbook, 2nd edition please CLICK HERE.
For additional information on anything contained in this newsletter call 908/685-2333 or e-mail sales@leistritz-extrusion.com
Your Leistritz team,
Leistritz Extrusion
175 Meister Ave. Somerville, NJ, 08876, USA
ph: 908/685-2333
email: sales@leistritz-extrusion.com