
The following is included in this e-tech newsletter
- Modular, water-cooled dies introduced: Now available in Leistritz USA process laboratory for testing
- Influence of screw design and processing parameters on residence time using twin screw extruders
- Injection of sCO2 both expands & enhances the range of dosage forms
- Current state of development for melt extrusion and continuous granulation via twin screw extrusion
- Leistritz expands USA process development laboratory: Rubber extruder now available for tests
- Know-how update: Pharmaceutical Extrusion Technology Textbook, 2nd edition

ZSE-18 with MWC model die front-end attachment
Model MWC die expands and improves range of extruded products
Leistritz' model MWC (modular water-cooled) dies have been integrated with twin screw extruders to extrude various Texturized Vegetable Protein (TVP) formulations in response to the growing demand for alternative proteins as a substitute for traditional animal-based meat diets. The MWC die extends the plant based “cooking” process and imparts texture into these alternative proteins. MWC dies also allow for unique geometric shapes for applications such as snack bars, dental sticks, animal chews and recreational gummies.
MWC dies are supplied in segments with internal cooling bores for liquid temperature control. Unit(s) can be plumbed together or utilized separately; water temperature control units (TCUs) can be utilized to modify cooling for more precise temperature profiles.
Additional features of the model MWC die design are that the modules can be stacked for extended cooling and on-line sensors can be integrated as dictated by the process. FTIR, NIR, raman and UVNN sensors can provide feedback for monitoring moisture, color and protein content; or chemical composition in case of reactive extrusion.
As we continue to expand our Life Science extrusion capabilities in the Leistritz Extrusion USA lab, MWC dies are now available. With six different extruder sizes, co and counter-rotating extruders, a myriad of solids and liquids feeders and various downstream sizing equipment, Leistritz offers the most comprehensive process development laboratory in North America for the advancement of Life Science products.
Influence of screw design and processing parameters on residence time using twin screw extruders
Recent studies have been performed to evaluate (and quickly estimate) the effect of screw design, screw rpms, and throughput rate on residence time (RT) and residence time distribution (RTD) in co-rotating intermeshing twin screw extruders (TSEs) for melt extrusion. RT provides insight into the exposure of thermally sensitive API’s and polymers to elevated temperatures during melt extrusion.
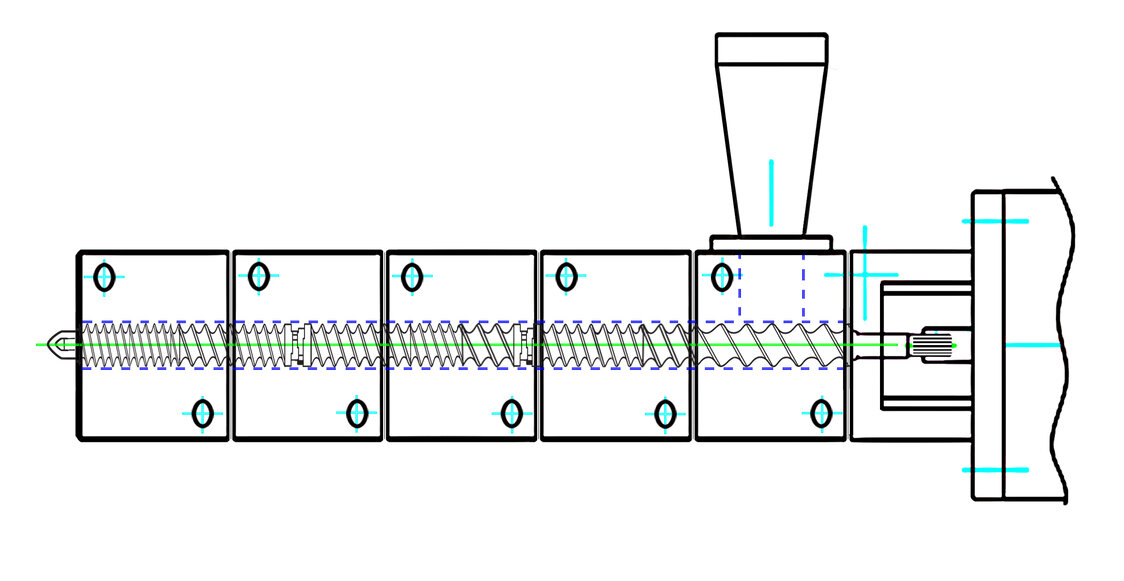
ZSE-18 process section used for residence time studies
Calculations for both filled and unfilled screw sections are utilized to help understand what the anticipated residence times are at various rates, rpms, and screw designs. Using these relatively simple calculations, which are meant to be insightful if not 100 % accurate, the residence time can be approximated and evaluated to help gauge the thermal exposure a formulation will experience in a TSE process section. Optimum hardware configurations and process parameters can then be inferred to minimize API degradation and improve processing efficiencies.
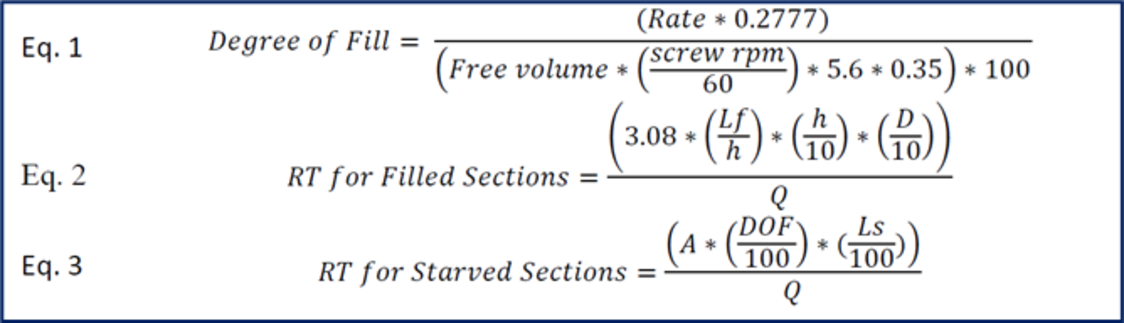
It is evident that as free volume in the TSE increases for a given rate, so will the RT. Increasing the feed rate will reduce residence time. As the screw speed increases with a constant rate, the RT will shorten, but not significantly, and the RTD will broaden. As the rate increases (with a constant screw speed) the RT will shorten and the RTD will tighten.
Different model TSEs were evaluated.
A Leistritz ZSE 27 MAXX model co-rotating TSE with 28.3 mm screws diameter at 40:1 L/D (1.66 OD/ID, 14.3 cc/dia free volume) and a Leistritz ZSE 18 HP co-rotating TSE with 18 mm screws diameter at 25:1 L/D (1.5 OD/ID, 3.2 cc/dia free volume) were compared to discern the residence times associated based on screw design and changes in process parameters.
Throughput rates and screw rpms were varied to see the effect on residence time. Additionally, processing temperatures were adjusted based on material properties and requirements. Color tracers were added to the main feed throat of all machines and the residence time was visually assessed. Trends were as expected, and no outliers were seen.
ZSE 18 HP calculated RTs:
| RPM | Rate (kg/hr) | RT (s) | RT (Calc) | RPM | Feed Rate (kg/hr) | RT (s) | RT (Calc) |
|---|---|---|---|---|---|---|---|
| 200 | 2 | 32 | 29.16 | 300 | 0.5 | 41 | 54.96 |
| 300 | 2 | 24 | 22.99 | 300 | 1 | 35 | 33.65 |
| 400 | 2 | 20 | 19.91 | 300 | 2 | 24 | 22.99 |
| 500 | 2 | 17 | 18.06 | 300 | 3 | 23 | 19.44 |
| 600 | 2 | 15 | 16.82 | 300 | 4 | 21 | 17.66 |
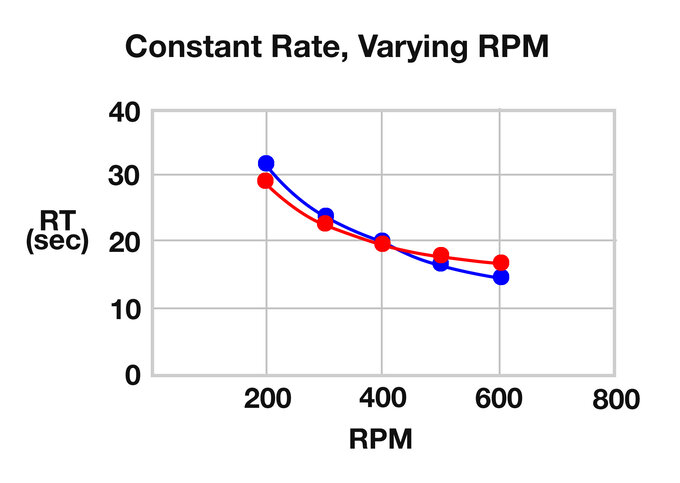
Constant Rate, Varying RPM
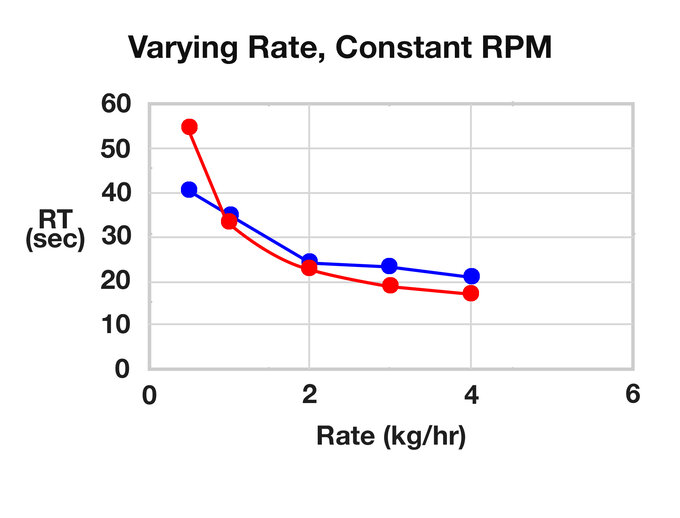
Varying Rate, Constant RPM
Quick overview of available twin screw extrusion technologies
Twin screw extruders are available for research and development purposes to process as little as a 50 gm batch, and for full-scale production at more than 50,000+ kgs/hr. Twin screw extruders utilize two screws that interact with each other, which makes the TSE useful for wide-ranging mass-transfer functions. Single screw extruders (SSEs) are used primarily to melt and pump plastics, while the TSE is deemed a superior tool for continuous mixing, devolatilization and reactive processing.
Twin screw extruders are available in these basic designs:
- Co-rotating intermeshing
- Counter-rotating intermeshing
- Counter-rotating non-intermeshing (or tangential)

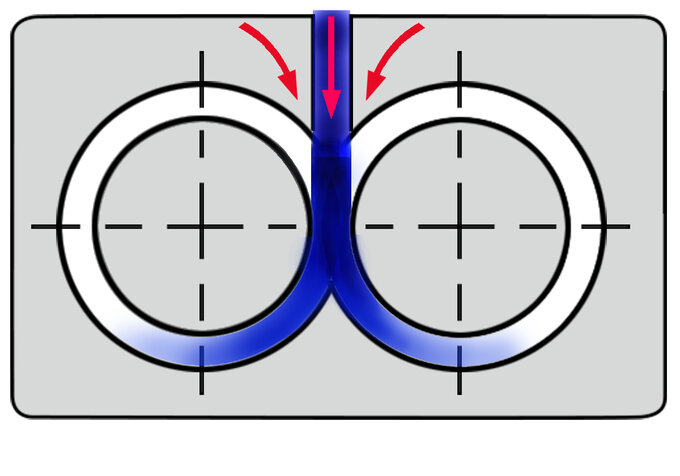
Example of a co-rotating intermeshing screw set

Example of a counter-rotating intermeshing screw set
Each type of TSE has technical merit. The co-rotating intermeshing mode is deemed best for most compounding applications. The low speed, late fusion counter-rotating intermeshing twin screw mode is desirable for low speed, late fusion processes. The tangential counter-rotating TSE is currently only used for specialty processes.
White paper: Quick overview of commercially available twin screw extruders (TSEs)
Injection of sCO2 both expands & enhances the range of dosage forms

Foamed pellets from melt extrusion process
Supercritical carbon dioxide (sCO2) is commonly injected into twin screw extruders in the plastics and food industries to facilitate foaming of high-rate, mass produced products. Significant development efforts are occurring that utilize TSE technologies as part of unique foam extrusion systems for emerging pharmaceutical and Life Science products.
The injection of sCO2into a TSE process section for use as a foaming agent improves the milling efficiency and tableting of amorphous solid dispersions (ASDs). Milling efficiency of the ASDs was significantly improved up to around 90 % increase in the mass recovery, while the tablet of the foamed and milled extrudates showed a considerable increase in corresponding tablet tensile strength.
The segmented nature of the TSE, in combination with controlled pumping and wiping characteristics of self-wiping co-rotating, intermeshing screws, allows the user to match screw and barrel geometries to the sequence of unit operations. The following describes likely sub-processes performed in a foam TSE process section.
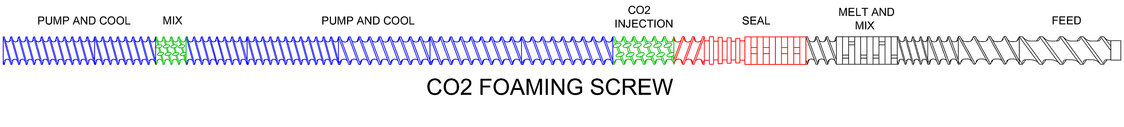
Example typical screw design for sCO2 injection/mixing
Metering multiple feed streams to the TSE: Raw materials are metered the TSE by loss-in-weight feeders and TSE screws speed (RPM) is used to optimize melting/mixing efficiencies.
Conveyance/melting/compounding: Solids conveying, melting and mixing occurs in the early part of the TSE process section. The materials must be thoroughly mixed and conditioned prior to the liquid/gas injection.
Injection/mixing/seal: After initial melting and mixing, sCO2 is injected into the TSE and mixed, just after a dynamic seal is achieved by the screw and barrel design.
Cooling/pumping: TSE screws now convey the melt forward. The dissolved sCO2 functions as a plasticizer, lowering Tg causing a viscosity decrease. Barrel temperature set-points are lowered to cool the melt and raise viscosity in preparation for final conditioning in a die.
Experiment: Indomethacin (INM) was used as a poorly-water soluble (BCS II) model drug and PVCap-PVAc-PEG as a model hydrophilic polymer. The experiments were performed utilizing a Leistritz ZSE-18 mm, 40 L/D, co-rotating TSE using sCO2 as a physical blowing agent. Foamed and un-foamed extrudates were milled and dissolution testing was performed. Cell and wall sizes of the foamed extrudates were measured from SEM images.
This study (and others) indicates that the sCO2 foam extrusion process can be successfully applied without adversely affecting the API within the polymer matrix while also improving of milling efficiencies and tablet-ability due to formation of porous and smaller particles during the milling process.
Overall, studies demonstrated that HPMCAS polymer is a suitable candidate for preparation of amorphous solid dispersion with robust milling property and higher compatibility with the foaming process, which facilitates a fast “dissolution-erosion” controlled release profile
Download: Supercritical CO2 Foam Extrusion (article in Journal of Pharmaceutical Sciences)
The current state of development for melt extrusion and continuous granulation via twin screw extrusion
Interest in extrusion for pharmaceutical products began in the 1980s, primarily in melt extrusion. Continuous granulation processes via twin screw extrusion are still in the embryonic stages of development.
Melt extrusion: The case study of Rezulin™, the first drug manufactured via melt extrusion, provides enlightening history. In 1989, Parke-Davis/Warner-Lambert converted a spray dried process to an alternative organic solvent-free manufacturing methodology, now referred to as Melt Extrusion (ME). The product was approved by the FDA and introduced in 1997, becoming the first commercial melt-extruded product. Due to the consistency and reproducibility of the process, the batch size was increased to 5,000 kilograms over 500 hours. During the time the product was in the market, there were no batch failures. A USA patent for the formulation and process was issued in 2004. Soon thereafter, in 2005, Abbvie received U.S. Food and Drug Administration (FDA) approval for another melt-extruded product, a novel formulation of an HIV protease inhibitor in tablet form.
The FDA’s Process Analytical Technology (PAT) in 2004 initiative provided drug makers a framework for pharmaceutical development, continuous processing and quality assurance through in-line monitoring that encourages that continuous operations (i.e. melt extrusion) be considered for the manufacture of new dosage forms. Activities relating to melt extrusion activity began to accelerate and the seeds had been planted for the current success story from the early work at Parke-Davis, and research institutions such as the University of Texas.
Download white paper:
Twin Screw Extruders as Continuous Mixers for Thermal Processing from a Historical Perspective
Leistritz Granulation Consortium
Developments for wet and melt granulation processes via twin screw extrusion have lagged behind melt extrusion. Hence, the Leistritz Granulation Consortium has been conceived and organized.
The concept of the Leistritz Granulation Consortium is simple. Leading representatives from industry and academia periodically meet to brainstorm ideas to advance granulation technologies, including but not limited to:
- Identify the existing challenges in twin-screw granulation and downstream processing
- Defining and performing experiments for twin screw granulation studies for publication
- Comparing twin screw granulation performance to existing technologies
- Identifying unique formulation/applications that can’t be processed by existing technologies
- New hardware developments to facilitate the success and expand use of twin screw granulation processes
The Leistritz Granulation Consortium is an open exchange forum where companies are encouraged to contribute as possible without divulging any proprietary information. Participants help identify industry issues and become part of the solution to advance granulation technologies from formulation, equipment and validation perspectives.
Participation in the consortium is by invitation and is non-binding. The first meeting of the consortium was held in May of 2019 at the Leistritz facility in Nuremberg, Germany, with subsequent (virtual) meetings held twice/year. Additional in-person meetings are planned for later in 2022. Participating companies include: Leistritz, Merck, AbbVie, McMaster University and University of Texas @ Austin.
One particular study that emanated from the consortium investigated the influence of binder addition techniques on continuous twin screw melt granulation. This study compared three binder addition methods in TSMG: (1) pre-mixing API and binder (pre-mixing), (2) feeding drug at the main port and liquid inject molten binder (liquid injection), and (3) feeding binder at the main port and side stuffing API (side stuffing). The objective was to examine the effect of binder addition methods on (1) granule formation, and (2) stability of primary solid particles. A ZSE-18 mm co-rotating twin screw extruder was used to prepare granules containing 80 % lactose monohydrate (LAC, solid particles) and 20 % hydroxypropyl cellulose (HPC, Klucel™ ELF grade, binder).
See the results as presented at the conference:
CRS 2021 Conference Presentation
For additional information about the Leistritz Granulation Consortium you are welcome to contact:
Brian Haight
Assistant Lab Manager, Leistritz Extrusion
E bhaight@leistritz-extrusion.com
Leistritz expands USA process development laboratory: Adds rubber extruder
The Leistritz laboratory facility has wide ranging capabilities including, but not limited, to run the following trials:
- Melt extrusion, melt granulation and wet granulation
- 50 gm batch sample to 100+ kgs/hr
- Transdermal/laminate systems, including coex tooling
- Supercritical CO2 injection for foaming, stripping or as a fugitive plasticizer
- Mono-layer or coex rods or tubes, cut-to-length or wound on reels
- Wide range specialty formulations and parts
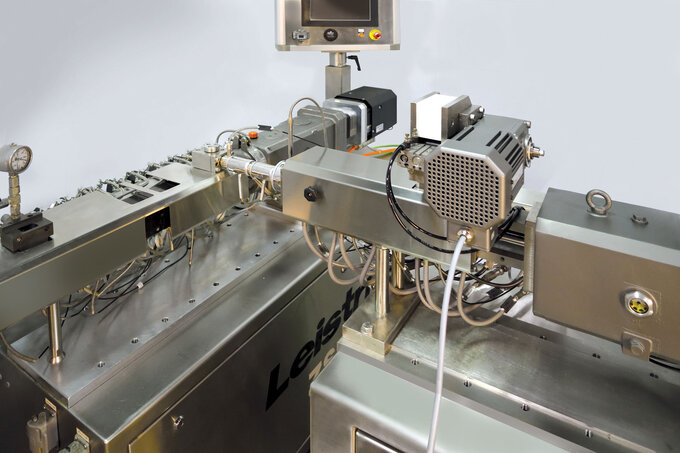
Rubber extruder to condition and a feed stream to the primary TSE
A unique capability that has been added is Leistritz model M-GG-27 counter-rotating twin screw extruder that accepts rubber strips and/or cubes and meters a conditioned rubber feed stream to a ZSE-compounding twin screw extruder in a controlled and repeatable manner.
Rubber strip feed mechanism
The M-GG-27 gearbox facilitates operation of the screws in an inward, counter-rotating mode of operation to facilitate feeding.
A 2-roll feed assembly can also be positioned at the M-GG-27 feed throat to facilitate metering of strips into the nip region of the screw in a consistent and repeatable manner.
Contact Leistritz today to discuss the development of the next breakthrough dosage form!
Know-how update: Pharmaceutical Extrusion Technology textbook, 2nd edition
Edited by Isaac Ghebre-Sellassie, Charles Martin, James DiNunzio and Feng Zhang
The first edition of Pharmaceutical Extrusion Technology, published in 2003, was deemed the seminal book on pharmaceutical extrusion. Now it is expanded and improved, just as the usage of extrusion has expanded, improved and evolved into an accepted manufacturing technology to continuously mix active pharmaceutical ingredients with excipients for a myriad of traditional and novel dosage forms.

Pharmaceutical Extrusion Technology Textbook, 2nd edition
Pharmaceutical Extrusion Technology (second edition, published in 2018) reflects how extrusion has spawned numerous research activities, in addition to hardware and process advancements. This edition now includes expanded chapters and contains all the extrusion related technical information necessary for the development, manufacturing, and marketing of pharmaceutical dosage forms.
Features include:
- Explanation as to why extrusion has become an accepted technology to continuously mix active pharmaceutical ingredients with excipients
- Focuses on equipment and process technology
- Describes various extrusion system configurations for a variety of dosage forms
- Presents new opportunities available only via extrusion and future trends
- Includes contributions of experts from the process and equipment fields
To review or to order Pharmaceutical Extrusion Technology Textbook, 2nd edition, please see:
We look forward to serving you in the future.
For additional information on anything contained in this newsletter please contact us
Team @ Leistritz Extrusion
Leistritz Extrusion Technology
175 Meister Ave. Somerville, NJ, 08876, USA
Tel: 908/685-2333
Email: sales@leistritz-extrusion.com
Website: extruders.leistritz.com